Active Implant Medical Devices Safety: EME Interferences, EMC Testing with Wavecontrol SMP3 Portable Monitoring Device
Active implant medical devices, AIMDs, are crucial devices for sustaining and improving the quality of life for many Australians with health conditions. These devices range from pacemakers and cardioverter-defibrillators to cochlear implants and even implantable devices for diabetic monitoring.
For many of these devices, rigorous testing is applied to ensure that they can sustain operation under conventional applications, and testing for interference from electromagnetic energy is no different.
Patients with active implant medical devices can be at risk due to the effects of low-frequency EME and pulsed high-frequency EME signals. This can pose a potential risk to patients with pacemakers and cardioverter-defibrillators, as it may create asynchronous pacing or, in the case of cardioverter-defibrillators, trigger an unwanted defibrillation shock.
For manufacturers of these devices, rigorous testing is necessary to ensure compliance with EMC certification standards, such as ISO 14117, IEC 60601-1-2, and CENELEC EN 45502-2-1. These certification standards ensure these devices are capable of functioning within a reasonable EMC exposure limit.
For Australian manufacturers of AIMD devices, learn more about the Australian Government's Therapeutic Goods Administration guidelines here.
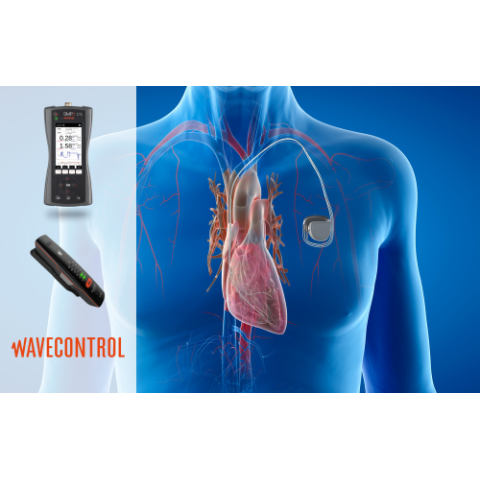
For manufacturers of these devices, the Wavecontrol SMP3 is a testing device more than capable of monitoring EME fields when testing AIMD devices meeting EMC certification standards. The SMP3 portable meter, paired with a WPH-DC probe, can monitor magnetic fields and low-frequency signals up to 10 MHz, encompassing the relevant frequency spectrum that AIMD patients can expect to encounter.

For AIMD patients who work or commute through areas with infrastructure producing EME, the WaveMon LF-400 is an ideal solution for monitoring personal risk. These devices feature configurable alarms to suit the intended exposure thresholds of the AIMD device and human exposure limit, enabling them to remove themselves from the environment when they are at risk.

If you are a provider of active implant medical devices in Australia and require an ILAC-accredited calibrated EMF monitoring device, the Wavecontrol SMP3 EMF portable meter is the perfect solution to ensure your products meet EMC certification standards.
If you have any questions, the ADM team is here to help! Please send any questions via our contact page here or call the team directly on 1300 236 467.